Our Research
How is a worm like a peanut?
One central aim of the lab is to understand how and why such an enormously diverse set of agonists – from macroscopic live worms to microscopic inert particles to soluble enzymes – all induce a type 2 (allergic) immune response. To answer this question, we are focusing on the early stages of type 2 responses, when the immune system first detects an agonist and must decide whether and how to respond. We use genetic mouse models paired with type 2 agonists such as allergens and parasitic worms (helminths) to study the cells and molecules that regulate the type 2 immune response.
What we know
It is well established that type 2 inflammation leads to profound changes in tissue physiology driven by the cytokine interleukin 13 (IL-13). Hallmarks of type 2 immunity include hypercontractility of smooth muscle and epithelial remodeling that gives rise to hyperplasia of mucus-secreting goblet cells. These changes are critical for clearance of worm infection in a process termed "weep and sweep", but to anyone suffering from allergies they are more familiar as a runny nose and sneezing.
In 2010, the discovery of group 2 innate lymphoid cells (ILC2s) was an important step forward in understanding the initiation of type 2 inflammation. ILC2s reside in the tissues where type 2 agonists are first encountered, produce large amounts of IL-13, and have been implicated in the early immune response to numerous helminths and allergens.
ILC2 inputs and outputs
Searching upstream
Although ILC2s are among the first immune cells activated during type 2 inflammation, they are not themselves the sentinels that detect type 2 stimuli. Instead, ILC2s respond to host-derived cytokines and lipids and integrate these signals to assess the state of their surrounding tissue and are activated by disruptions in tissue homeostasis that are common to the diverse set of type 2 agonists. We are therefore looking upstream to determine where these ILC2-activating signals come from and how they are regulated.
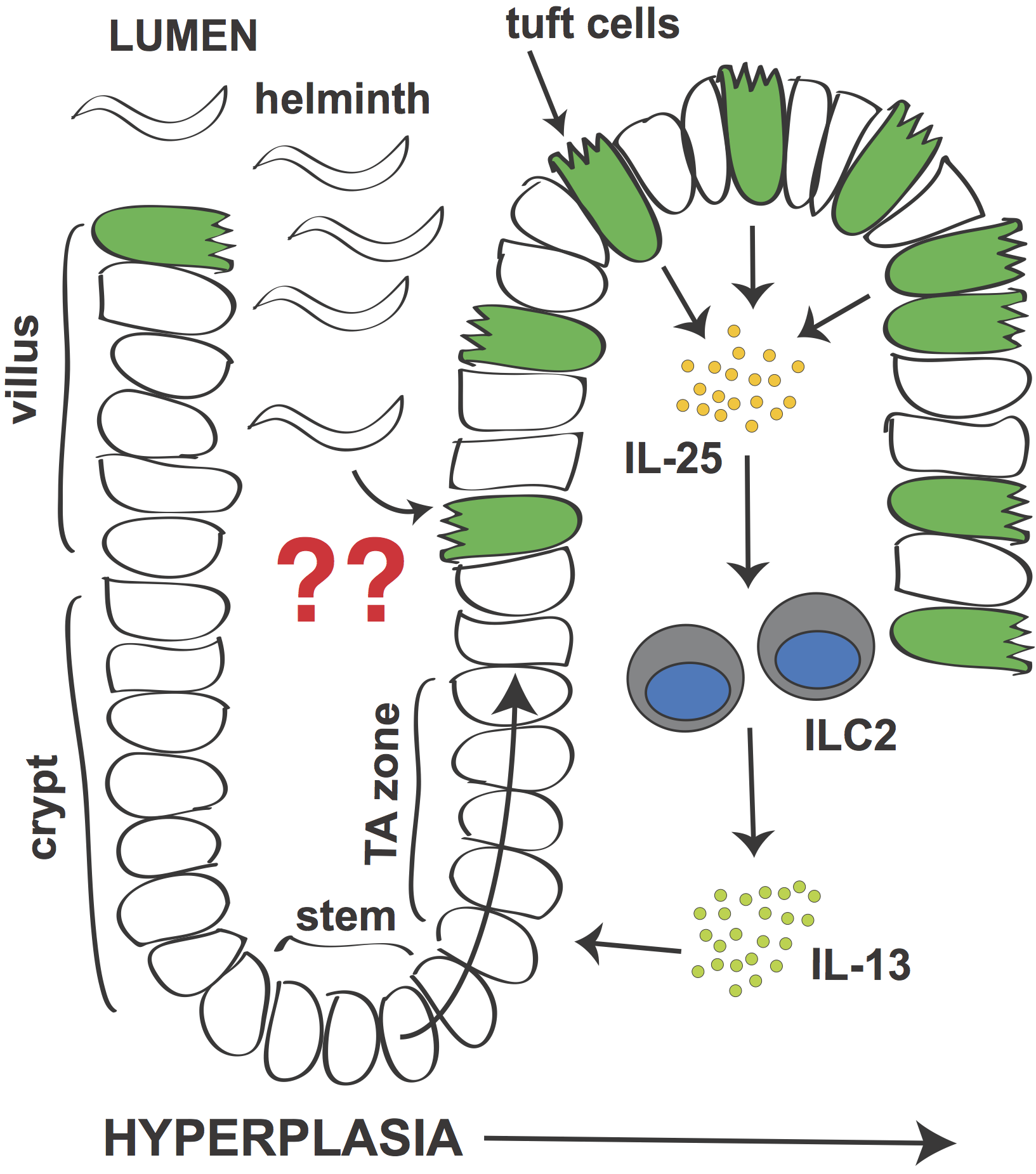
Signaling circuit in small intestine that leads to tuft cell hyperplasia during helminth infection.
Tuft cells: the missing link?
In 2016 we and others reported that a specialized epithelial lineage called tuft cells are required for the intestinal type 2 immune response. Although normally quite rare, during infection the number of tuft cells increases dramatically. This tuft cell hyperplasia (and the previously described goblet cell hyperplasia) is regulated by a signaling circuit in which tuft cells secrete IL-25 to induce IL-13 production by ILC2s. IL-13 in turn signals in epithelial stem cells to bias their lineage commitment towards tuft and goblet cells. Without tuft cells, epithelial remodeling fails and worm clearance is delayed.
"Tufts" of long apical microvilli give tuft cells their name.
Tuft cells (also sometimes called brush cells) take their name from a defining “tuft” of microvilli at the apical surface. Tuft cells were therefore first described more than 60 years ago when electron microscopy became common practice, but their physiologic function remained elusive. Our findings identified tuft cells as novel members of the type 2 immune system, and we believe that they represent the missing link between type 2 agonists in the lumen and immune cells in the underlying tissue. Not only are they ideally positioned to perform this function, tuft cells also encode components of the canonical taste transduction pathway, suggesting a role in type 2 immune sensing. Indeed, type 2 inflammation is attenuated and worm clearance is delayed when this chemosensing pathway is disrupted.
Long microvilli of a tuft cell extend past the brush border of neighboring epithelial cells.
There is much to learn about the intriguing biology of tuft cells, and they are currently the primary focus of the lab. In particular, we hope to identify a tuft cell-encoded receptor and the cognate ligand that underlie sensing of parasitic worms. We are also interested in the tuft cell effector functions that contribute to type 2 immunity and perhaps to other physiologic processes.